Tuning the Solvation Structure in Aqueous Zinc Batteries to Maximize Zn-Ion Intercalation and Optimize Dendrite-Free Zinc Plating
Abstract
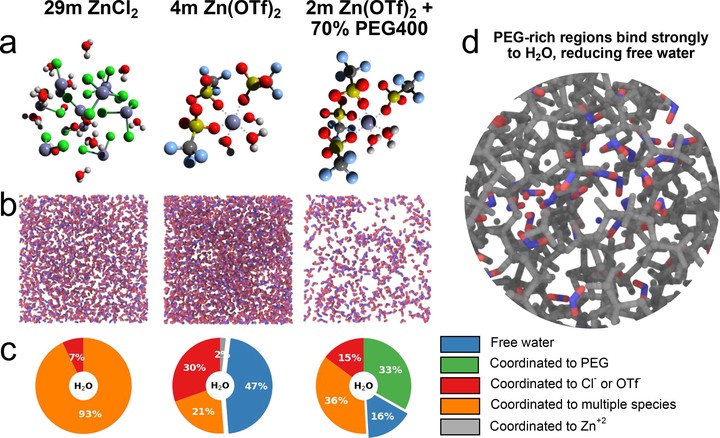
Aqueous zinc batteries are recognized to suffer from H/Zn coinsertion in the cathode, but few approaches have been reported to suppress deleterious H+ intercalation. Herein, we realize this goal by tuning the solvation structure, using LiV (LVP) as a model cathode. Phase conversion of LVP induced by H+ intercalation is observed in 4 m Zn(OTf), whereas dominant Zn2+ insertion is confirmed in a ZnCl2 water-in-salt electrolyte (WiSE). This disparity is ascribed to the complete absence of free water and a strong Zn–HO interaction in the latter that interrupts the H2O hydrogen bonding network, thus suppressing H+ intercalation. On the basis of this strategy, a novel PEG-based hybrid electrolyte is designed to replace the corrosive ZnCl WiSE. This system exhibits an optimized Zn$^{2+} solvation sheath with a similar low free water content, showing not only much better suppression of H+ intercalation but also highly reversible Zn plating/stripping with a CE of ∼99.7% over 150 cycles.