Partitioning of Alkali Metal Salts and Boric Acid from Aqueous Phase into the Polyamide Active Layers of Reverse Osmosis Membranes
Abstract
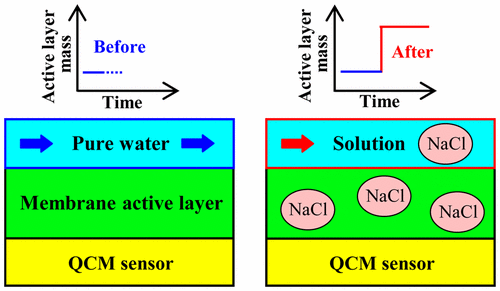
The partition coefficient of solutes into the polyamide active layer of reverse osmosis (RO) membranes is one of the three membrane properties (together with solute diffusion coefficient and active layer thickness) that determine solute permeation. However, no well-established method exists to measure solute partition coefficients into polyamide active layers. Further, the few studies that measured partition coefficients for inorganic salts report values significantly higher than one (∼3–8), which is contrary to expectations from Donnan theory and the observed high rejection of salts. As such, we developed a benchtop method to determine solute partition coefficients into the polyamide active layers of RO membranes. The method uses a quartz crystal microbalance (QCM) to measure the change in the mass of the active layer caused by the uptake of the partitioned solutes. The method was evaluated using several inorganic salts (alkali metal salts of chloride) and a weak acid of common concern in water desalination (boric acid). All partition coefficients were found to be lower than 1, in general agreement with expectations from Donnan theory. Results reported in this study advance the fundamental understanding of contaminant transport through RO membranes, and can be used in future studies to decouple the contributions of contaminant partitioning and diffusion to contaminant permeation.